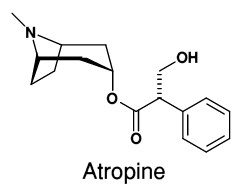
This is a quick post about a chemical that saved one of my favourite characters on TV last weekend. It was a syringe of the chemical atropine administered fortuitously to Quinn before his exposure to sarin gas that meant he lived to tell the tale ! Atropine, or (RS)-(8-Methyl-8-azabicyclo[3.2.1]oct-3-yl) 3-hydroxy-2-phenylpropanoate as it is systematically known as, is a compound found in the deadly nightshade plant. Isolated from the plant back in 1833, it now has a variety of medical uses including in the treatment of Parkinson’s disease, maintaining regular heart function during surgery and even treating scorpion stings. There is a really interesting article about the synthesis of atropine in the link below. Richard Willstätter completed the synthesis in 1901, and it demonstrates the challenges faced by the organic chemists of that era who had to identify their product by carrying out chemical tests and comparing to the natural product rather than analysis using spectroscopic techniques available today.
So how did this molecule save Quinn ? Sarin is an organophosphate nerve agent. It acts by inhibiting the enzyme acetylcholinesterase. Acetylcholine is a neurotransmitter activating nerve fibres that cause muscles to contract. Sarin forms a covalent bond with a residue at the active site on the enzyme making it unable to bind to acetylcholine. This means the acetylcholine molecule is not broken down after it activates the fibres, the delicate balance of acetylcholine and its degradation products is disrupted, and acetylcholine builds up and continues to make the nerves connect over and over and over again. How does atropine work ? It blocks the acetylcholine receptor stopping it from working in the first place. This is not ideal as it means that the nerves are not driving muscle function but it stops seizures and convulsions.
So thanks to atropine I can sit down tomorrow night to another instalment of Homeland knowing that one of the CIAs most prolific spies is back in action and may still save the day !



